New drugs are constantly being developed for breast cancer. In the future, artificial intelligence will be used to assess individual risk for cancer. Liquid biopsy, on the other hand, reveals the risk of cancer recurrence.
Breast cancer is the most common cancer in women. In Finland, around 5,000 women are diagnosed with breast cancer every year, and one in eight women will get breast cancer at some point in their lives. However, the prognosis of a patient in Finland is one of the best in Europe: 85% of those with breast cancer will be alive ten years after the diagnosis.
Breast cancer is not one single disease, but a group of different subtypes. Based on the biological properties of the tumour, breast cancer is divided into luminal hormone-dependent breast cancer, HER2-positive breast cancer or triple negative breast cancer.
Most cases of breast cancer are hormone dependent and slow-growing
In hormone-dependent breast cancer, there are hormone receptors on the surface of cancer cells to which the female hormones oestrogen and progesterone are bound, thus contributing to cancer growth. This cancer type is the most common, usually the slowest to progress, and the one with best prognosis. In HER2 positive breast cancer, there is an abundance of HER2 growth factor receptors on the cell surface, promoting cancer growth. In triple negative breast cancer, there are no hormone or HER2 receptors on the cancer cell surface. HER2 positive and triple negative breast cancer are typically aggressive; however, HER2 positive breast cancer can nowadays be treated with effective precision drugs.
“HER2 antibodies have been a major improvement in the treatment of this type of cancer. More and more of them have been introduced in the past 5–10 years, and they are nowadays routinely used in treatment,” says Päivi Auvinen, Service Director and Chief of Department at the Cancer Centre of Kuopio University Hospital.
Breast cancer is primarily treated by surgery. When necessary, surgery is followed by radiation therapy and drugs, i.e., cytostatics that destroy cancer cells and, depending on the cancer type, also hormone therapy that blocks the action of female hormones, or precision drugs. Sometimes, drug therapy is initiated already before surgery to, for example, improve tumour operability.
Pre-surgery drug therapy is known as neoadjuvant treatment.
“For the treatment of HER2 positive breast cancer, for example, we have several new drugs whose effectiveness has been studied specifically before surgery, so they will be used in hospitals as neoadjuvant treatment for larger or more widespread tumours. They are an interesting addition to treatment, although actual comparative studies before versus after surgery are unfortunately scarce. The clinical impression nevertheless is that neoadjuvant treatment would be associated with fewer cases of cancer recurrence.”
Immunotherapy requires good collaboration and communication with the patient.
Päivi Auvinen

Immunotherapy turns the body’s own immune defence against cancer cells
“Pre-surgery immuno-oncology, on the other hand, is bringing a significant change to the treatment of triple negative breast cancer. Previously, immuno-oncology was only used in the treatment of cancer that had already spread, but now it can be used to start treatment in cases where the tumour is large or extending to the armpit,” Auvinen says.
Immuno-oncology, also known as immunotherapy, helps the patient’s immune system to identify cancer cells and to attack them as enemies.
“Immunotherapy can be targeted at immunologically active cancers. Of the different types of breast cancer, only triple negative breast cancer is immunologically active. In a best-case-scenario, neoadjuvant treatment can eliminate breast cancer completely, but longer-term treatment outcomes are still being studied.”
Immunotherapy doesn’t necessarily cause any other side effects than fatigue. In some patients, however, it can make the immune system to attack healthy organs, possibly leading to, for example, skin, liver or gastrointestinal inflammation, heart symptoms or diabetes even months after treatment.
“This is why immunotherapy requires good collaboration and communication with the patient. The patient’s family and other health care professionals, too, must understand the link between immunotherapy and various possible symptoms.”
In the treatment of hormone-dependent breast cancer, no similar innovations are on the horizon. However, genetic profiling of the tumour is a new opening in the planning of treatment. It can be used to get a more accurate assessment of the risk of cancer recurrence and, consequently, of the need for cytostatics, so that burdensome treatments are not initiated in vain.
Precision drugs for tumour mutations
When necessary, genetic profiling of the cancer tumour can also be used to select a suitable drug if, for example, standard treatment chosen according to the biological cancer type doesn’t yield the desired results.
“If cancer becomes resistant to treatment, an analysis of the genetic mutations of the tumour may provide insight into the type of drug that could be effective,” says Professor Arto Mannermaa of the Institute of Clinical Medicine at the University of Eastern Finland.
In recent years, multi-professional Molecular Tumour Boards (MTB) have been established in large hospitals to plan the treatment of individual patients based on genetic profiling of their tumour. However, since genetic profiling is expensive, it is currently performed for a small number of patients only.
“At Kuopio University Hospital, the MTB began its work recently, and it is also linked to the activities of FICAN East and the university’s multidisciplinary cancer research community.”
“Genetic profiling can be purchased from commercial service providers abroad, but analytics can also be performed at the university, in which case researchers can be involved in interpreting the results and making their expertise also otherwise available to treatment planning.”
Cancer develops when mutations occur in the genome of cells, disrupting their normal function and causing them to divide uncontrollably. As tumour cells divide, they continue to mutate, which means that the multitude of genetic mutations may be different at different stages of cancer development, and there may also be internal variation in the tumour.
“The significance of mutations found in genetic tumour profiling, and drugs possibly already found to be effective against them, can be studied in international, ever-expanding databases. However, data on all mutations is not readily available. Together with Professor Antti Poso’s research group, we will be exploring them with the help of computer modelling,” Mannermaa says.
Based in the School of Pharmacy at the University of Eastern Finland, the research group is capable of modelling the effects of a mutation and virtually testing all existing pharmaceutical ingredients for their effectiveness against that particular mutation. Poso also works as a visiting professor at the University of Tübingen in Germany, where this expertise is already being used to choose treatments for cancer patients in the university hospital.
We need a simple method for monitoring people who have had breast cancer and for predicting cancer recurrence. Liquid biopsy could be a feasible tool.
Arto Mannermaa

Liquid biopsy helps predict the recurrence of breast cancer
“There are nearly 80,000 women in Finland who have had breast cancer, and in 20–30% of them, cancer will recur. We need a simple method for monitoring people who have had breast cancer and for predicting cancer recurrence. Liquid biopsy could be a feasible tool and it is currently being studied extensively,” Mannermaa says.
In liquid biopsy, cancer biomarkers are searched in bodily fluids, such as blood samples. Mannermaa’s research group has shown, e.g., that breast cancer patients with a poor prognosis can be identified based on the degree of breakdown of circulating extracellular DNA released by the tumour.
An interesting finding was that this method could be used to identify patients with a poor prognosis from among patients with hormone-positive breast cancer, which is traditionally considered to have a good prognosis.
In another study, the research group showed that breast cancer recurrence could be predicted by liquid biopsy from a blood sample months before the recurrence was detected during a medical examination. This was based on cancer mutations in extracellular DNA.
“Our next endeavour is to use liquid biopsy to isolate living, circulating cancer cells. Circulating cancer cells may play an important role in the formation of metastases. Analysing them may shed light on the progression of cancer. In the laboratory, the idea is to test pharmaceutical ingredients targeting the mutations they carry.”
In Finland, liquid biopsy is not yet routinely used, but according to Mannermaa, it may in the future supplement traditional methods in the diagnosis, prognosis assessment and monitoring of breast cancer, too.
Circulating biomarkers are also the topic of an ongoing study examining how extracellular vesicles, i.e., messengers excreted into their surroundings by cancer cells, can promote resistance to treatment.
“Cells that have been given radiation therapy seem to use vesicles to sound an alarm of a kind to other cells, possibly contributing to the development of resistance to treatment. We are now interested in how treatment changes the composition of vesicles, and what kind of cellular signalling pathways are affected.”
Machine learning enables the identification of patient groups that may benefit from certain treatments.
Hamid Behravan

Artificial intelligence may reveal individual cancer risk
“Most cancers are caused by coincidence, when ageing cells undergo a sufficient number of mutations that affect cell function,” Mannermaa says.
Besides age, many other factors, which affect the risk of breast cancer, are also known. An estimated 5–10% of cases are caused by genetic mutations that significantly increase the risk of breast cancer, such as mutations of the BRCA or PALB2 genes. There are various additional genetic factors that could raise the risk of breast cancer, alongside changes in gene regulation that can be influenced by lifestyle choices and environmental factors.
The amount of lifetime exposure to oestrogen affects at least the risk of hormone-positive breast cancer, and for example overweight and heavy drinking may increase the risk by influencing the body’s oestrogen level.
In Finland, mammography is used to regularly screen women aged 50–69 for breast cancer.
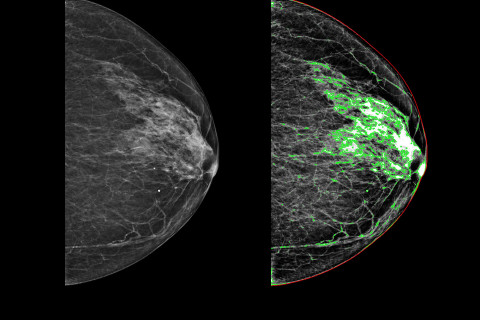
“In addition to screening for cancer, mammography images can also be used to assess the risk of breast cancer later in life. Dense breast tissue is associated with an increased risk of breast cancer.”
More detailed information on the real individual risk can be obtained by means of artificial intelligence and machine learning methods that can simultaneously examine a wide range of risk factors and their interactions. These methods are being developed by Senior Researcher Hamid Behravan, together with Mannermaa.
“For example, the genetic risk for breast cancer has previously been assessed on the assumption that different factors affect the risk independently of each other. However, we have shown that by taking their interactions into account, we can achieve an increasingly accurate risk assessment,” Behravan says.
The machine learning model developed by the group has identified genetic regions that are essential to the risk of breast cancer, and that these regions also interact with one another. The work helps to locate risk genes and to understand interactions between genes underlying the risk of cancer.
“By combining this with data on oestrogen metabolism and hereditary risk for breast cancer, we could predict cancer even more reliably.”
The results also helped to identify gene networks linked to breast cancer, which are associated with, for example, tumour growth, cell division and DNA repair mechanisms.
Behravan’s goal is to introduce a multi-factor risk assessment tool for health care professionals, which would enable the identification of both high- and low-risk individuals for breast cancer, allowing screening and monitoring to be adjusted accordingly.
“This would also help to avoid unnecessary imaging and costs.”
Artificial intelligence can also be used to interpret imaging results. The research group has developed an AI-based method for automated estimation of breast tissue density from mammography images. The method provides uniform estimates of tissue density, which may facilitate the work of radiologists, while also contributing to density assessment training.
“The reliability of this method has already been tested with thousands of mammography images obtained from Kuopio University Hospital, and the plan is to test it also in international imaging data.”
In the future, the research group strives to develop solutions not only for risk assessment but also for prognosis assessment in breast cancer patients.
“Machine learning enables the identification of patient groups that may benefit from certain treatments, for example.”
All about cancer: Breast cancer. https://www.kaikkisyovasta.fi/tietoa-syovasta/syopataudit/rintasyopa/
Koivunen JP, Iivanainen S & Karihtala P. Kohdennetut syövän hoidot - häviääkö syöpäkirurgia? Duodecim 2020;136(13):1547-51. https://www.duodecimlehti.fi/duo15686
Loponen H, Barker H, Ylisaukko-Oja T, Bärlund M& Tanner M. Rintasyövän neoadjuvanttihoidon toteutuminen ja tulokset. Suom Lääkäril 2022; 78 : e33517. https://www.laakarilehti.fi/tieteessa/alkuperaistutkimukset/rintasyovan-neoadjuvanttihoidon-toteutuminen-ja-tulokset/
Finnish Breast Cancer Group. National recommendation for the diagnostics and treatment of breast cancer 2023. https://1587667.167.directo.fi/@Bin/7538651e1999006dfbd2b5193b4dd956/1677828712/application/pdf/193311/SRSR__Suositus%202023_2.pdf
Tiainen L & Utriainen M. Rintasyövän ennustetekijät täsmentyvät. Duodecim 2022;138:307–14. https://www.duodecimlehti.fi/duo16707
Vihinen P, Mattila K, Mäkelä S, Hernberg M & Koivunen J. Immuno-onkologisten lääkkeiden käyttö, haittavaikutukset ja niiden hoito. Duodecim 2019;135(21):2095-103. https://www.duodecimlehti.fi/duo15225
Behravan H, Hartikainen JM, Tengström M. et al. Predicting breast cancer risk using interacting genetic and demographic factors and machine learning. Sci Rep 10, 11044 (2020). https://doi.org/10.1038/s41598-020-66907-9
Gudhe NR, Behravan H, Sudah M. et al. Area-based breast percentage density estimation in mammograms using weight-adaptive multitask learning. Sci Rep 12, 12060 (2022). https://doi.org/10.1038/s41598-022-16141-2